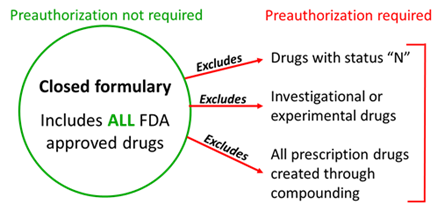
The formulary consists of all available Food and Drug Administration (FDA) approved prescription and nonprescription drugs prescribed and dispensed for outpatient use, with the following exclusions:
- drugs identified with a status of "N" in the current edition of the Official Disability Guidelines Treatment in Workers' Comp (ODG) / Appendix A, ODG Workers' Compensation Drug Formulary, and any updates;
- any prescription drug created through compounding prescribed and dispensed; and
- any investigational or experimental drug for which there is early, developing scientific or clinical evidence demonstrating the potential efficacy of the treatment, but which is not yet broadly accepted as the prevailing standard of care as defined in Labor Code §413.014(a).
Preauthorization is required for any drugs excluded from the formulary.