
The pharmaceutical benefits rules in Title 28 of the Texas Administrative Code §134.500 through §134.550 apply to claims subject to certified workers' compensation health care networks (network claims) and claims not subject to certified networks (non-network claims).
These rules include coverage for initial pharmaceutical services, prescribing generic and brand name drugs and over the counter alternatives, a closed formulary, a pharmacy fee guideline, and other requirements for pharmaceutical services. Understanding the rules for pharmacy services and related resources is fundamental for prescribing clinically appropriate medication and proper reimbursement.
- §134.500. Definitions. Includes key definitions pertaining to all pharmaceutical benefit rules. Amended April 2018.
- §134.501. Initial Pharmaceutical Coverage. Provides for payment of pharmaceutical services sufficient for the first seven days following the date of injury.
- §134.502. Pharmaceutical Services. Requires doctors to prescribe generics and over-the-counter alternatives when appropriate.
- §134.503. Pharmacy Fee Guideline. Concerns the reimbursement of pharmaceutical services for brand name and generic drugs which are based on the average wholesale price times a multiplier. Details of reimbursement methodologies for compounds and over-the-counter medications are described in this rule.
- §134.504. Pharmaceutical Expenses Incurred by the Injured Employee. Provides a process for the claimant to obtain reimbursement for medications that have been purchased out-of-pocket.
- §134.506. Outpatient Open Formulary for Claims with Dates of Injury Prior to September 1, 2011. Addresses the formulary that applies to "legacy claims."
- §134.510. Transition to the Use of the Closed Formulary for Claims with Dates of Injury Prior to September 1, 2011. Addresses transition to the closed formulary for "legacy claims."
- §134.520. Outpatient Closed Formulary for Dates of Injury On or After September 1, 2011. Applies the closed formulary to dates of injury September 1, 2011, and forward.
- §134.530. Requirements for Use of the Closed Formulary for Claims Not Subject to Certified Networks. Describes the requirements for use of the closed formulary for non-network claims. Amended April 2018.
- §134.540. Requirements for Use of the Closed Formulary for Claims Subject to Certified Networks. Describes the requirements for use of the closed formulary for certified network claims. Amended April 2018.
- §134.550. Medical Interlocutory Order, or MIO. Provides a means for an injured employee to continue use of the previously prescribed and dispensed drug(s) throughout the duration of the appeals/dispute process after a preauthorization denial. A pharmacy or prescribing doctor can submit a request for an MIO using DWC Form-064, Medical Interlocutory Order Request.
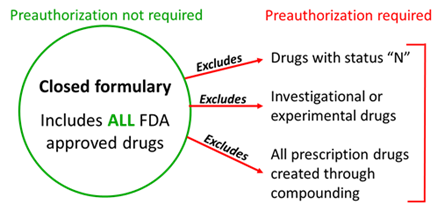
The formulary consists of all available Food and Drug Administration (FDA) approved prescription and nonprescription drugs prescribed and dispensed for outpatient use, with the following exclusions:
- drugs identified with a status of "N" in the current edition of the Official Disability Guidelines Treatment in Workers' Comp (ODG) / Appendix A, ODG Workers' Compensation Drug Formulary, and any updates;
- any prescription drug created through compounding prescribed and dispensed; and
- any investigational or experimental drug for which there is early, developing scientific or clinical evidence demonstrating the potential efficacy of the treatment, but which is not yet broadly accepted as the prevailing standard of care as defined in Labor Code §413.014(a).
Preauthorization is required for any drugs excluded from the formulary.
Preauthorization rules
The Division of Workers' Compensation (DWC) provides Appendix A, ODG Workers' Compensation Drug Formulary, from the ODG in a convenient format for quick reference only. It is not a substitute for the current edition of Appendix A: ODG Workers' Compensation Drug Formulary or any reference to the Texas Labor Code or DWC rules.
Monthly updates to Appendix A, ODG Workers' Compensation Drug Formulary
Appendix A in the online edition of the ODG is updated as new evidence becomes available. DWC will update the listing in the Appendix A spreadsheet monthly as new information is received from ODG. However, the online edition of the ODG is the official source for DWC actions.
ODG Appendix A (Excel)
28 TAC §134.503, Pharmacy Fee Guideline applies to reimbursement of prescription drugs and nonprescription drugs or over-the-counter medications as defined in §134.500 (relating to Definitions) for outpatient use in the Texas workers' compensation system, which includes:
- network claims;
- non-network claims; and
- certain government employee workers’ compensation claims (Labor Code §504.053(b)(2).
Note: The pharmacy fee guideline does not apply to parenteral drugs. Parenteral drugs are reimbursed using the methodology in the Medical Fee Guideline for Professional Services [28 TAC, §134.203(d)] for non-network claims, or by a workers’ compensation network contract for network claims.
Insurance carriers and their agents, workers’ compensation networks, and government employers are prohibited from requiring that injured employees receive pharmacy services from a specific pharmacy. Injured employees may fill prescriptions at the pharmacy of their choice if the pharmacy accepts workers' compensation.
Texas Labor Code
- §408.028. Pharmaceutical Services.
- §408.0281. Reimbursement for Pharmaceutical Services; Administrative Violation.
- §504.053. Election. (b)(2).
Health care provider training and resources
On demand videos (YouTube playlist)
Live webinars (webinar schedule)
Other resources
